The Heriot-Watt Astrochemistry Research Group |
 |
Reflection-Absorption Infrared Spectroscopy (RAIRS / IRRAS)

Infrared radiation is used to probe vibrational molecular motion. Only those vibrations whose dipole moment lies perpendicular to the metal surface can be observed. This is because the incident and reflected p-polarised components of the radiation superimpose constructively (add together), enhancing the signal, whereas the s-polarised components cancel each other out, (as they undergo a phase change on reflection from the metal surface).
RAIR spectra show bulk and surface features. These are used to characterise the structure of the ice and the ice surface, and to evaluate the nature of the bonds between molecules in the ices, as well as the between ice molecules and the adsorbate molecules.
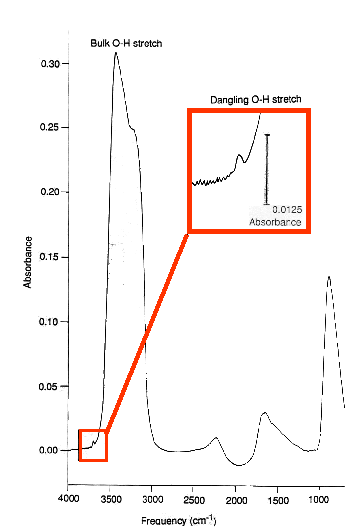
Dangling OH bonds at Ice Surfaces
In water ices one of the most distinguishing spectral features is the OH stretching vibration. The bulk OH stretching vibration dominates an ice spectrum. On the shoulder of this feature a second signal exists, (see spectrum below) which is attributable to the OH bonds that are dangling from the surface of the ice (those that are not involved in the hydrogen bonding network). In a thick ice sample this feature is quite weak as there are far fewer OH bonds at the surface compared with the bulk. It is also a more distinctive feature than that of bulk OH, since the OH itself is less tightly bound in the lattice.In water ice the dangling OH stretch appears at:
- 3692 cm-1 in crystalline ice
- 3696 cm-1 in 3-coordinate ice
- 3720 cm-1 in 2-coordinate ice
In this experiment the IR spectrum is recorded using a Fourier Transform (FT) spectrometer, which encompasses a 60o Michelson Interferometer. Interferometeric techniques greatly enhance the speed and sensitivity at which this type of spectrocopy is conducted.
The IR data ties in with IR spectra obtained by the astronomical community in observational astronomy. They have already observed ice features in astronomical spectra taken in lines-of-sight towards reddened stars, where visible light has been obscured by dust.
The FTIR spectrometers were purchased from:

Biorad